Research
The group members have more than 20 years long experience in the development of advanced recombinant biomaterials applied in different biomedical fields. Our materials, inspired by natural proteins, are engineered taking advantage of molecular design and biosynthesis technology, and the group research covers the physicochemical characterization of the products to their evaluation in applications both in vitro and in vivo.
This family of functionalized and tailored biopolymers responds to the needs of modern biomaterials, such as high biocompatibility, bioactivity and functionalization potential, fully controlled complex composition, and stimuli responsiveness.
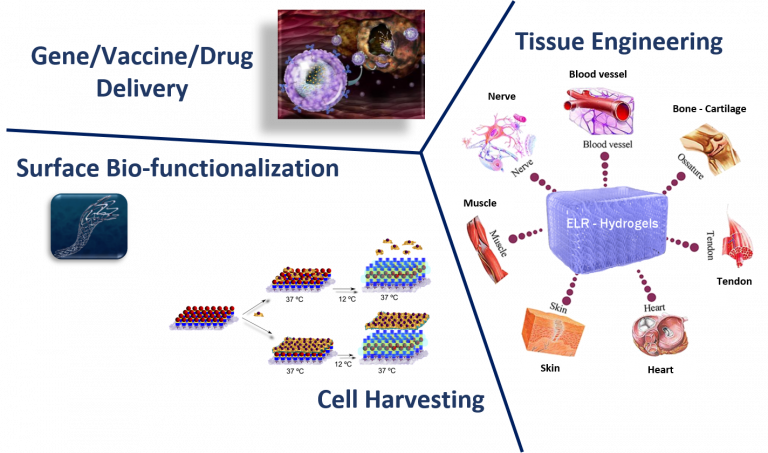
Many collaborations with professionals in the field contributed to gathering deeper insight into the characteristics of our materials, highlighted their versatility, and brought them to high-impact publications. Among the various areas of interest, basic research focused on the understanding of materials properties, tissue engineering, and gene and drug delivery.
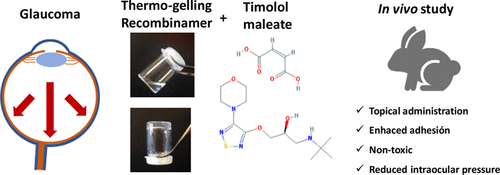
Injectable hydrogels based on our biomaterials have been developed from single-molecule self-gelling devices or two-component by covalent crosslink with relevant in vivo results in drug delivery (Fernández-Colino, et al., Materials Sci. & Engineering C, 2016; Mol. Pharmaceutics, 2017; Moreno-Estar, et al., Acta Biomat. 2020; Pepe, et al., ACS Biomat. Sci. Eng., 2021; González et al., Pharmaceutics, 2022).
Regarding Nanomedicine, several alternative branches of the field have been explored. On one hand, immunomodulatory nanoparticles from single biopolymer molecules and DNA-derived nanovaccines were successfully tested in vivo (García-Arévalo, et al, Mol. Pharmaceutics, 2013; González-Valdivieso, et al., Mol. Pharmaceutics, 2020; Girotti, et al., Methods in Molecular Biology, 2021). On other hand, gene delivery strategies employing our biomaterials demonstrated the suitability of the nanodevices also in the area of polyplex delivery for breast cancer treatment (Piña, et al., Mol Pharmaceutics, 2016; Cancer Lett., 2020).
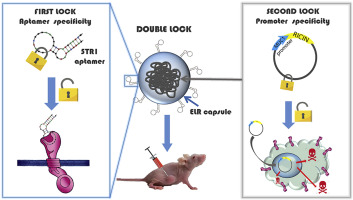
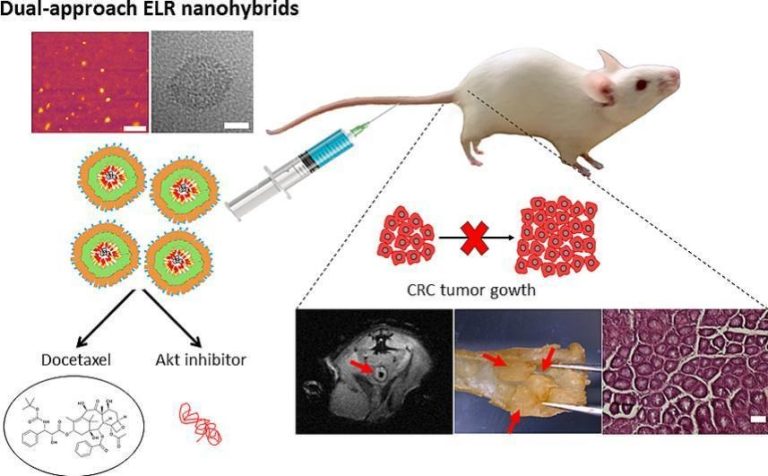
Recently, a smart kind of Nanoparticles has been designed and tested for targeted drug delivery in in vitro tumor cells and in vivo animal models for cancer treatment. This family of nanodevices can be selectively directed to tumor cells by peptidic or aptamer molecules and they can transport highly hydrophobic anti-cancer drugs or peptidic inhibitors to modulate cellular growth by inactivation of Akt kinase (González-Valdivieso, et al, Biomacromol., 2019; Vallejo, et al., J. Ind. & Eng. Chem., 2021; González-Valdivieso, et al., ACS App. Mat. Interf., 2021; Biomat. Adv. 2023; Vallejo, et al., Int. J. Pharmaceutics, 2024).
Additionally, several review articles coped with kinetic control aspects and model drugs tested that pave the way for the future application of our protein-based systems for drug-delivery purposes (Arias, et al., Curr. Op. Medicinal Chem., 2014; Curr. Drug Targets, 2018; Santos, et al., Curr. Organic Chem, 2017; Curr. Medicinal Chem., 2020; Girotti, et al., Pharmaceutics, 2020; González-Valdivieso, et al., Int. J. Pharmaceutics, 2021; Muñoz, et al., Cancers, 2021).
Finally, the group leader has also contrastable experience in Antibody Fragment development and biosynthesis for 4 years (Franconi, et al. Immunotechnology, 1999; Tavladoraki, et al. FEBS Journal, 1999) and in recombinant protein expression and characterization (Arias, et al., IJBCB 2003; Antolín, et al., J. Biotechnol., 2004; Barriuso, et al. Toxins, 2016).