History
2019
““Self-Assembling ELR-Based Nanoparticles as Smart Drug- Delivery Systems Modulating Cellular Growth via Akt”
J. Gonzalez-Valdivieso, A. Girotti, R. Muñoz, J. C. Rodriguez-Cabello, F.J. Arias.
Biomacromolecules, 20, 1996-2007 (2019)
Impact Factor (JCR): 6.092 DOI: 10.1021/acs.biomac.9b00206
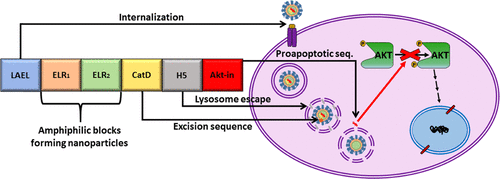
This work investigates the physicochemical properties and in vitro accuracy of a genetically engineered drug-delivery system based on elastin-like block recombinamers. The DNA recombinant techniques allowed us to create this smart complex polymer containing bioactive sequences for internalization, lysosome activation under acidic pH, and blockage of cellular growth by a small peptide inhibitor. The recombinant polymer reversibly self-assembled when the temperature was increased above 15 °C into nanoparticles with a diameter of 72 nm and negative surface charge. Furthermore, smart nanoparticles were shown to enter in the cells via clathrin-dependent endocytosis and properly blocked phosphorylation and consequent activation of Akt kinase. This system provoked apoptosis-mediated cell death in breast and colorectal cancer cells, which possess higher expression levels of Akt, whereas noncancerous cells, such as endothelial cells, fibroblasts, and mesenchymal stem cells, were not affected. Hence, we conclude that the conformational complexity of this smart elastin-like recombinamer leads to achieving successful drug delivery in targeted cells and could be a promising approach as nanocarriers with bioactive peptides to modulate multiple cellular processes involved in different diseases.
“Genetically Engineered Elastin-based Biomaterials for Biomedical Applications”
M. Santos, S. Serrano-Dúcar, J. González-Valdivieso, R. Vallejo, A. Girotti, P. Cuadrado, F.J. Arias
Current Medicinal Chemistry, 26, 7117-7146 (2019)
Impact Factor (JCR): 2.642 DOI: 10.2174/0929867325666180508094637
Protein-based polymers are some of the most promising candidates for a new generation of innovative biomaterials as recent advances in genetic-engineering and biotechnological techniques mean that protein-based biomaterials can be designed and constructed with a higher degree of complexity and accuracy. Moreover, their sequences, which are derived from structural protein-based modules, can easily be modified to include bioactive motifs that improve their functions and material-host interactions, thereby satisfying fundamental biological requirements. The accuracy with which these advanced polypeptides can be produced, and their versatility, self-assembly behavior, stimuli-responsiveness and biocompatibility, means that they have attracted increasing attention for use in biomedical applications such as cell culture, tissue engineering, protein purification, surface engineering and controlled drug delivery. The biopolymers discussed in this review are elastin-derived protein-based polymers which are biologically inspired and biomimetic materials. This review will also focus on the design, synthesis and characterization of these genetically encoded polymers and their potential utility for controlled drug and gene delivery, as well as in tissue engineering and regenerative medicine

2018
“Elastin-like recombinamers as smart drug delivery systems”
F.J. Arias, M. Santos, A. Ibáñez-Fonseca, MªJ. Piña, S. Serrano-Dúcar
Current Drug Targets, 19, 360-379 (2018)
Impact Factor (JCR): 2.642 DOI: 10.2174/1389450117666160201114617
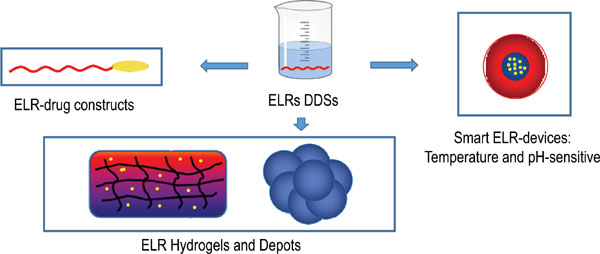
Drug delivery systems that are able to control the release of bioactive molecules and designed to carry drugs to target sites are of particular interest for tissue therapy. Moreover, systems comprising materials that can respond to environmental stimuli and promote self-assembly and higher order supramolecular organization are especially useful in the biomedical field. Objetive: This review focuses on biomaterials suitable for this purpose and that include elastin-like recombinamers (ELRs), a class of proteinaceous polymers bioinspired by natural elastin, designed using recombinant technologies. The self-assembly and thermoresponsive behaviour of these systems, along with their biodegradability, biocompatibility and well-defined composition as a result of their tailormade design, make them particularly attractive for controlled drug delivery. ELR-based delivery systems that allow targeted delivery are reviewed, especially ELR-drug recombinant fusion constructs, ELR-drug systems chemically bioconjugated in their monomeric and soluble forms, and drug encapsulation by nanoparticle-forming ELRs. Subsequently, the review focuses on those drug carriers in which smart release is triggered by pH or temperature with a particular focus on cancer treatments. Systems for controlled drug release based on depots and hydrogels that act as both a support and reservoir in which drugs can be stored will be described, and their applications in drug delivery discussed. Finally, smart drug-delivery systems not based on ELRs, including those comprising proteins, synthetic polymers and non-polymeric systems, will also be briefly discussed. Several different constructions based on ELRs are potential candidates for controlled drug delivery to be applied in advanced biomedical treatments.
2017
“Self-assembling Elastin-like hydrogels for timolol delivery: development of an ophthalmic formulation against glaucoma”
A. Fernández-Colino, D. Quinteros, D. Allemandi, A. Girotti, S. Palma, F.J. Arias
Molecular Pharmaceutics, 14, 4498-4508 (2017)
Impact Factor (JCR): 4.556 DOI: 10.1021/acs.molpharmaceut.7b00615
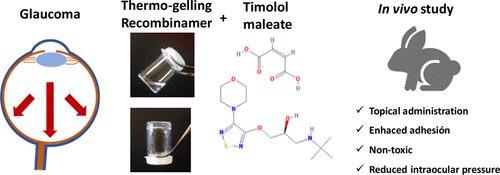
This work focuses on improving the effectiveness of current therapies against glaucoma by incorporating self-assembled polymers into the ophthalmic formulation. To that end, we first studied the influence of the dispersing medium on the mechanical performance of self-assembling elastin-like (EL) and silk-elastin-like (SEL) hydrogels by conducting rheological tests. These polymers were subsequently incorporated into the antiglaucoma formulation, which contains timolol maleate (TM) as active ingredient, and in vivo tests, namely adhesion tests and intraocular pressure measurements (IOP), were performed in New Zealand rabbits. An enhanced reduction in IOP due to the presence of the polymers was observed. Moreover, differences in the effectiveness between both EL- and SEL-hydrogels, which can be explained on the basis of the different rheological properties displayed by these two systems, were also encountered. The results point to the potential of this system as a basis for the development of an ophthalmic formulation against glaucoma.
“Advanced systems for controlled drug delivery from chemically modified Elastin-like Recombinamers”
M. Santos, C. González-Obeso, D. Orbanic, E. Pérez del Río, F.J. Arias
Current Organic Chemistry, 21: 21-33 (2017)
Impact Factor (JCR): 2.193 DOI: 10.2174/1385272820666160511120925
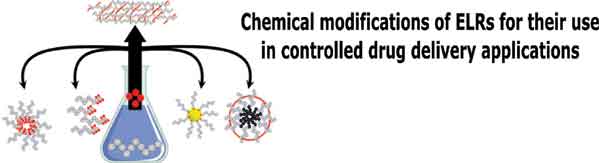
Targeted drug delivery is a new multidisciplinary field that aims to develop innovative nanomaterials, tools and devices to deploy a therapeutic agent to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. Advanced drug-delivery systems attempt to control the site of action and release rate and act by means of either a physiological or a chemical trigger. In this sense, stimuliresponsive biomaterials are of special interest for application as components of drug-delivery devices. This review discusses the use of elastin-like recombinamers (ELR) in drug-delivery systems. These biopolymers possess special properties that encompass biodegradability, bioactivity and stimuli-responsiveness. Their tailormade design using recombinant DNA technologies allows an absolute control of their amino acid sequence and design of the most appropriate macromolecule for each application. Firstly, devices based on monomeric elastinlike recombinamers which have been chemically modified to attach functionalities that enable us to follow or direct their distribution or anticancer drugs in an attempt to improve drug-conjugate uptake are described. Secondly, ELRs that form part of nanoparticles as drug carriers will be studied in their different versions, including nanoparticles chemically reinforced by interchain cross-linking, nanoparticles formed by self-assembly of chemically modified ELRs to achieve amphiphilic properties and multifunctional composites made up of nanoparticles coated with ELRs. Finally, recent advances in the area of 3D platforms for drug delivery, comprising interconnected hydrogels and ELR-based coacervates in the form of depots, will be reviewed.
2016
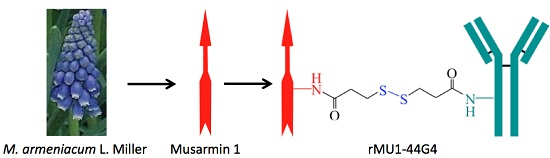
“Anti-human Endoglin (hCD105) Immunotoxin Containing Recombinant Single Chain Ribosome-inactivating Protein Musarmin 1”
B. Barriuso, P. Antolín, F. J. Arias, A. Girotti, P. Jiménez, M. Córdoba-Diaz, D. Córdoba-Diaz, T. Girbés
Toxins, 8: E184 (2016)
Impact Factor (JCR): 3.273 DOI: 10.3390/toxins8060184
Endoglin (CD105) is an accessory component of the TGF-β receptor complex, which is expressed in a number of tissues and over-expressed in the endothelial cells of tumor neovasculature. Targeting endoglin with immunotoxins containing type 2 ribosome-inactivating proteins has proved an effective tool to reduce blood supply to B16 mice tumor xenografts. We prepared anti-endoglin immunotoxin (IT)—containing recombinant musarmin 1 (single chain ribosome-inactivating proteins) linked to the mouse anti-human CD105 44G4 mouse monoclonal antibody via N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP). The immunotoxin specifically killed L929 fibroblast mouse cells transfected with the short form of human endoglin with IC50 values in the range of 5 × 10−10 to 10−9 M.
“Biocompatible ELR based polyplexes coated with MUC1 specific aptamers and targeted for breast cancer gene therapy”
M.J. Piña, A. Girotti, M. Santos, J.C. Rodríguez-Cabello, F.J. Arias
Molecular Pharmaceutics, 13: 795-808 (2016)
Impact Factor (JCR): 4.440 DOI: 10.1021/acs.molpharmaceut.5b00712
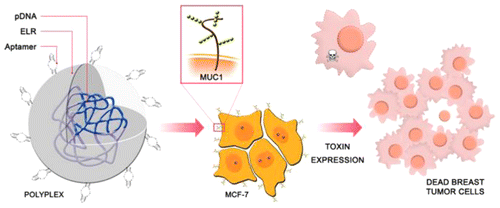
The search for new and biocompatible materials with high potential for improvement is a challenge in gene delivery applications. A cell type specific vector made of elastin-like recombinamer (ELR) and aptamers has been specifically designed for the intracellular delivery of therapeutic material for breast cancer therapy. A lysine-enriched ELR was constructed and complexed with plasmid DNA to give positively charged and stable polyplexes. Physical characterization of these polyplexes showed a particle size of around 140 nm and a zeta potential of approximately +40 mV. The incorporation of MUC1-specific aptamers into the polyplexes resulted in a slight decrease in zeta potential but increased cell transfection specificity for MCF-7 breast cancer cells with respect to a MUC1-negative tumor line. After showing the transfection ability of this aptamer-ELR vector which is facilitated mainly by macropinocytosis uptake, we demonstrated its application for suicide gene therapy using a plasmid containing the gene of the toxin PAP-S. The strategy developed in this work about using ELR as polymeric vector and aptamers as supplier of specificity to deliver therapeutic material into MUC1-positive breast cancer cells shows promising potential and continues paving the way for ELRs in the biomedical field.
“Development of a mechanism and an accurate and simple mathematical model for the description of drug release: application to a relevant example of acetazolamide-controlled release from a bio-inspired elastin-based hydrogel”
A. Fernández Colino, J. M. Bermúdez, F. J. Arias, D. A. Quinteros, E. E. Gonzo
Materials Science & Engineering C-Materials for Biological Applications, 61: 286-292 (2016)
Impact Factor (JCR): 4.164 DOI: 10.1016/j.msec.2015.12.050t.5b00712
Transversality between mathematical modeling, pharmacology, and materials science is essential in order to achieve controlled-release systems with advanced properties. In this regard, the area of biomaterials provides a platform for the development of depots that are able to achieve controlled release of a drug, whereas pharmacology strives to find new therapeutic molecules and mathematical models have a connecting function, providing a rational understanding by modeling the parameters that influence the release observed. Herein we present a mechanism which, based on reasonable assumptions, explains the experimental data obtained very well. In addition, we have developed a simple and accurate “lumped” kinetics model to correctly fit the experimentally observed drug-release behavior. This lumped model allows us to have simple analytic solutions for the mass and rate of drug release as a function of time without limitations of time or mass of drug released, which represents an important step-forward in the area of in vitro drug delivery when compared to the current state of the art in mathematical modeling. As an example, we applied the mechanism and model to the release data for acetazolamide from a recombinant polymer. Both materials were selected because of a need to develop a suitable ophthalmic formulation for the treatment of glaucoma. The in vitro release model proposed herein provides a valuable predictive tool for ensuring product performance and batch-to-batch reproducibility, thus paving the way for the development of further pharmaceutical devices.