Publications
2025
“Effective ocular delivery of antioxidant polyphenols using elastin-like polymer nanosystems developed by sustainable process“
L. Krstić, R. Vallejo, S. Rodríguez-Rojo, M.J. González-García, F.J. Arias, A. Girotti, Y. Diebold
International Journal of Pharmaceutics, 125691 (2025)
Impact Factor (JCR): 5.3 DOI: 10.1016/j.ijpharm.2025.125691
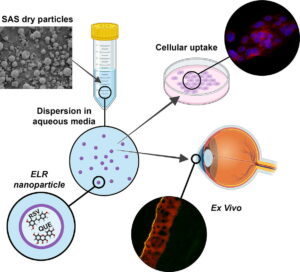
The present study is centred on the development of a therapeutic strategy for Dry Eye Disease (DED), and all experimental models, formulation designs, and outcome measures have been specifically tailored to address this pathology. Elastin-like polymers (ELPs) are emerging as a groundbreaking nanobiomaterial for advanced drug delivery systems, thanks to their biomimetic, adaptable, and stimuli-responsive properties. Embracing sustainability, we employed supercritical CO2 (scCO2) as an eco-friendly alternative to organic solvents to develop an innovative ELP-based particulate system. This system, designed for topical ophthalmic applications, incorporates two antioxidant and anti-inflammatory polyphenolic compounds, quercetin (QUE) and resveratrol (RSV), through a one-step supercritical antisolvent (SAS) process. Post-SAS process, we achieved solid microparticles loaded with QUE, RSV, or a combination of both. Remarkably, these microparticles transform into nanoparticles (NPs) with an average size of 56.7 ± 1.0 to 61.5 ± 2.6 nm when placed in solution at physiological temperature. This transformation leverages the stimulus-responsive nature of ELP, ensuring sustained polyphenol release. Our ELP-based formulations demonstrate exceptional biocompatibility with Human Corneal Epithelial cells (HCEs) and exhibit outstanding intracellular scavenging activity against reactive oxygen species (ROS). To track cellular uptake, we developed particles with a two fluorescent tags. This system successfully delivered the fluorescent payload to cells and efficiently targeted the corneal epithelium in ex vivo porcine eye globes, showcasing time-dependent delivery.
2024
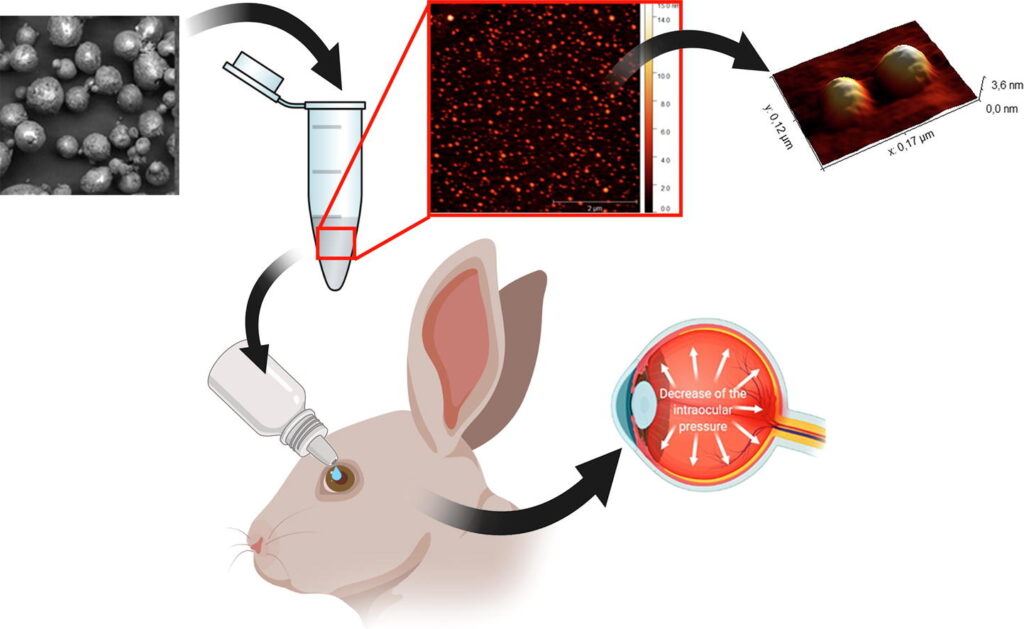
“Acetazolamide encapsulation in elastin like recombinamers using a supercritical antisolvent (SAS) process for glaucoma treatment“
R. Vallejo, D. Quinteros, J. Gutiérrez, S. Martínez, S. Rodríguez-Rojo, L.I. Tártara, S. Palma, F.J. Arias
International Journal of Pharmaceutics, 124098 (2024)
Impact Factor (JCR): 5.3 DOI: 10.1016/j.ijpharm.2024.124098
Glaucoma, the second most common cause of blindness worldwide, requires the development of new and effective treatments. This study introduces a novel controlled-release system utilizing elastin-like recombinamers (ELR) and the Supercritical Antisolvent (SAS) technique with supercritical CO2. Acetazolamide (AZM), a class IV drug with limited solubility and permeability, is successfully encapsulated in an amphiphilic ELR at three different ELR:AZM ratios, yielding up to 62 %. Scanning electron microscopy (SEM) reveals spherical microparticles that disintegrate into monodisperse nanoparticles measuring approximately 42 nm under physiological conditions. The nanoparticles, as observed via Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM), do not exhibit aggregates, a fact confirmed by the zeta potential displaying a value of –33 mV over a period of 30 days. Transcorneal permeation tests demonstrate a 10 % higher permeation level compared to the control solution, which increases to 30 % after 2 h. Ocular irritation tests demonstrate no adverse effects or damage. Intraocular pressure (IOP) tests conducted on hypertensive rabbits indicate greater effectiveness for all three analyzed formulations, suggesting enhanced drug bioavailability during treatment. Consequently, the combination of recombinant biopolymers and high-pressure techniques represents a promising approach for advancing glaucoma therapy, emphasizing its potential clinical significance.
2023
“CD44-targeted nanoparticles for co-delivery of docetaxel and an Akt inhibitor against colorectal cancer“
J. Gonzalez-Valdivieso, R. Vallejo, S. Rodriguez-Rojo, M. Santos, J. Schneider, F.J. Arias, A. Girotti
Biomaterials Advances, 213595 (2023)
Impact Factor (JCR): 7.9 DOI: 10.1016/j.bioadv.2023.213595
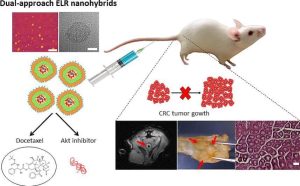
New strategies to develop drug-loaded nanocarriers with improved therapeutic efficacy are needed for cancer treatment. Herein we report a novel drug-delivery nanosystem comprising encapsulation of the chemotherapeutic drug docetaxel (DTX) and recombinant fusion of a small peptide inhibitor of Akt kinase within an elastin-like recombinamer (ELR) vehicle. This combined approach is also precisely targeted to colorectal cancer cells by means of a chemically conjugated DNA aptamer specific for the CD44 tumor marker. This 53 nm dual-approach nanosystem was found to selectively affect cell viability (2.5 % survival) and proliferation of colorectal cancer cells in vitro compared to endothelial cells (50 % survival), and to trigger both apoptosis- and necrosis-mediated cell death. Our findings also show that the nanohybrid particles remain stable under physiological conditions, trigger sustained drug release and possess an adequate pharmacokinetic profile after systemic intravenous administration. In vivo assays showed that these dual-approach nanohybrids significantly reduced the number of tumor polyps along the colorectal tract in a murine colorectal cancer model. Furthermore, systemic administration of advanced nanohybrids induced tissue recovery by improving the morphology of gastrointestinal crypts and the tissue architecture. Taken together, these findings indicate that our strategy of an advanced dual-approach nanosystem allows us to achieve successful controlled release of chemotherapeutics in cancer cells and may have a promising potential for colorectal cancer treatment.
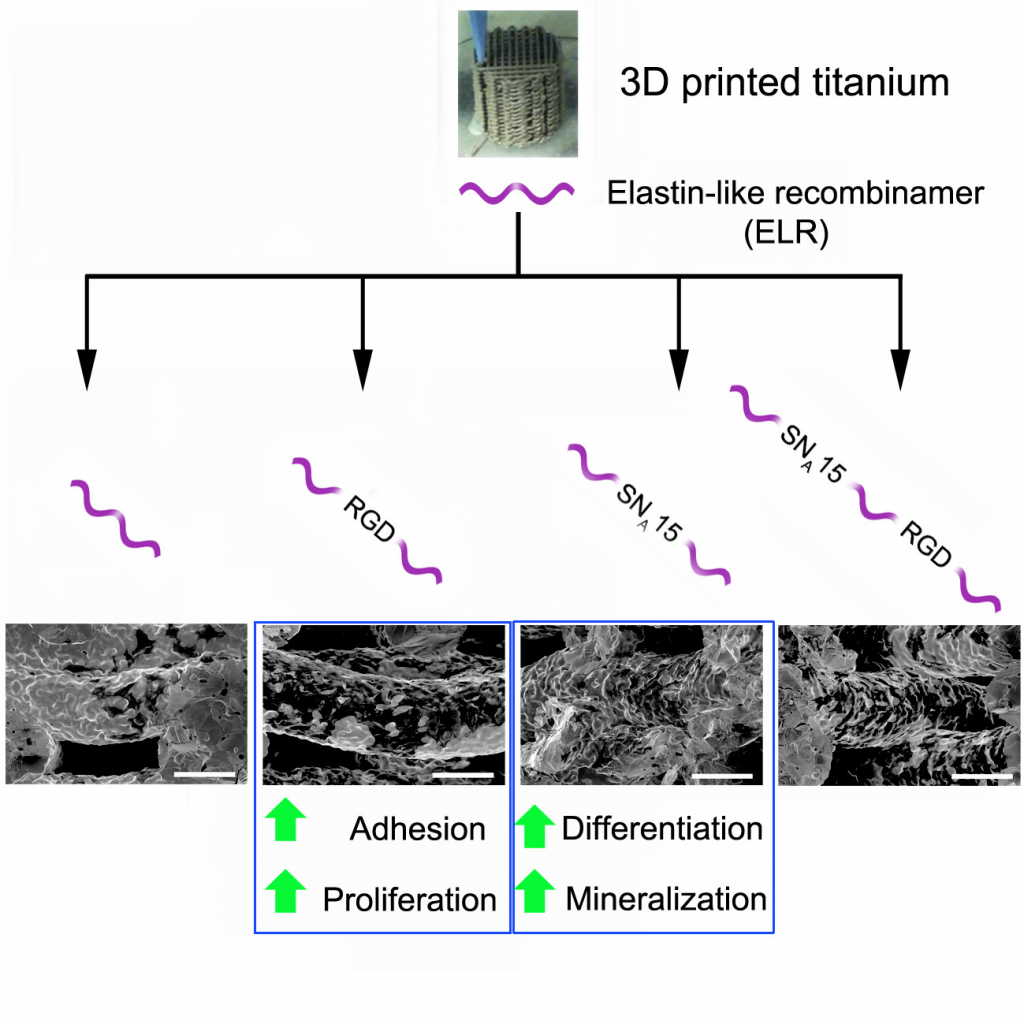
“Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction“
J. Guillem-Marti, E. Vidal, A. Girotti, A. Heras-Parets, D. Torres, F.J. Arias, M.P. Ginebra, J.C. Rguez-Cabello, J.M. Manero
Pharmaceutics, 15(3), 872 (2023)
Impact Factor (JCR): 4.9 DOI: 10.3390/pharmaceutics15030872
The 3D printing of titanium (Ti) offers countless possibilities for the development of personalized implants with suitable mechanical properties for different medical applications. However, the poor bioactivity of Ti is still a challenge that needs to be addressed to promote scaffold osseointegration. The aim of the present study was to functionalize Ti scaffolds with genetically modified elastin-like recombinamers (ELRs), synthetic polymeric proteins containing the elastin epitopes responsible for their mechanical properties and for promoting mesenchymal stem cell (MSC) recruitment, proliferation, and differentiation to ultimately increase scaffold osseointegration. To this end, ELRs containing specific cell-adhesive (RGD) and/or osteoinductive (SNA15) moieties were covalently attached to Ti scaffolds. Cell adhesion, proliferation, and colonization were enhanced on those scaffolds functionalized with RGD-ELR, while differentiation was promoted on those with SNA15-ELR. The combination of both RGD and SNA15 into the same ELR stimulated cell adhesion, proliferation, and differentiation, although at lower levels than those for every single moiety. These results suggest that biofunctionalization with SNA15-ELRs could modulate the cellular response to improve the osseointegration of Ti implants. Further investigation on the amount and distribution of RGD and SNA15 moieties in ELRs could improve cell adhesion, proliferation, and differentiation compared to the present study.
2022
“Silk-Elastin-Like Polymers for Acute Intraparenchymal
Treatment of the Traumatically Injured Spinal Cord: A First Systematic Experimental Approach“
P. González, C. Glez-Fernández, A. Maqueda, V. Pérez, S. Escalera-Anzola,
A. Rguez de Lope, F.J. Arias, A. Girotti and F.J. Rodríguez
Pharmaceutics, 14(12), 2713 (2022)
Impact Factor (JCR): 5.4 DOI: 10.3390/pharmaceutics14122713
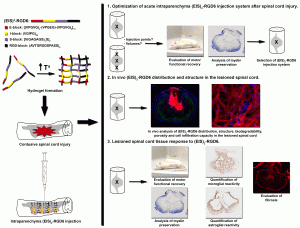
Despite the promising potential of hydrogel-based therapeutic approaches for spinal cord injury (SCI), the need for new biomaterials to design effective strategies for SCI treatment and the outstanding properties of silk-elastin-like polymers (SELP), the potential use of SELPs in SCI is currently unknown. In this context, we assessed the effects elicited by the in vivo acute intraparenchymal injection of an SELP named (EIS)2-RGD6 in a clinically relevant model of SCI. After optimization of the injection system, the distribution, structure, biodegradability, and cell infiltration capacity of (EIS)2-RGD6 were assessed. Finally, the effects exerted by the (EIS)2-RGD6 injection—in terms of motor function, myelin preservation, astroglial and microglia/macrophage reactivity, and fibrosis—were evaluated. We found that (EIS)2-RGD6 can be acutely injected in the lesioned spinal cord without inducing further damage, showing a widespread distribution covering all lesioned areas with a single injection and facilitating the formation of a slow-degrading porous scaffold at the lesion site that allows for the infiltration and/or proliferation of endogenous cells with no signs of collapse and without inducing further microglial and astroglial reactivity, as well as even reducing SCI-associated fibrosis. Altogether, these observations suggest that (EIS)2-RGD6—and, by extension, SELPs—could be promising polymers for the design of therapeutic strategies for SCI treatment.
“Elastin-like Polymers as Nanovaccines: Protein Engineering of Self-Assembled, Epitope-Exposing Nanoparticles»
A. Girotti, J. Gonzalez-Valdivieso, I. Alonso-Sampedro, S. Escalera-Anzola, S. Ramos-Díez, F.J. Arias
Methods in Molecular Biology, 2465, 41-72 (2022)
Vaccine Technologies for Veterinary Viral Diseases
DOI: 10.1007/978-1-0716-2168-4_3
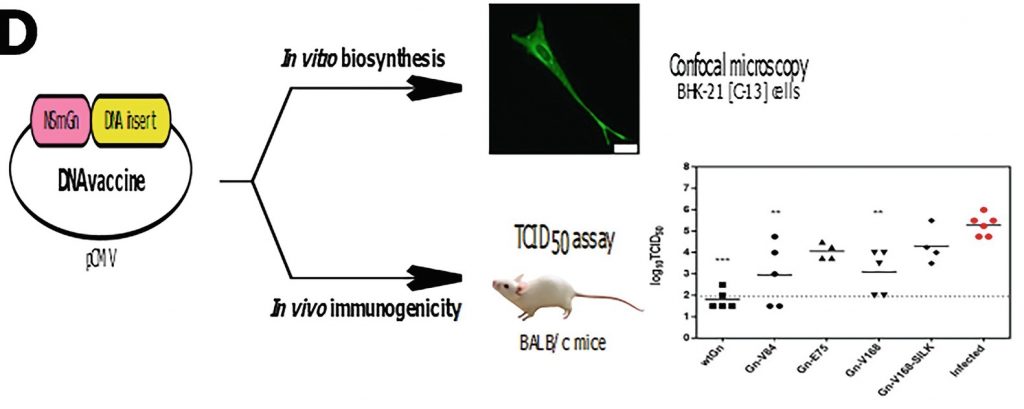
In this chapter we describe two unconventional strategies for the formulation of new nanovaccines. Both strategies are based on obtaining chimeric genes that code for proteins in which the major antigens of the pathogens are fused to an elastin-like recombinamer (ELR) as carrier. ELRs are a family of synthetic protein biopolymers obtained using DNA recombinant techniques. The ELRs employed in the present chapter are block copolymers that are able to assemble, under controlled conditions, into nanoparticles similar to virus-like particles and to provoke an immune response. We describe the biosynthesis of ELRs genetically fused to an antigenic sequence from Mycobacterium tuberculosis and a simple procedure for obtaining stable nanoparticles displaying the antigen in the first strategy. The second approach describes the production of a DNA vaccine library consisting of plasmids codifying for major antigens from Rift Valley fever virus fused to different ELR-based block copolymer architectures.
The procedures described can be adapted for the production of other chimeric DNA-protein vaccines based on protein polymer carriers.
2021
“Smart nanoparticles as advanced anti-Akt kinase delivery systems for pancreatic cancer therapy“
J. Gonzalez-Valdivieso, A. Garcia Sampedro, A. Hall, A. Girotti, F.J. Arias, S. Pereira, P. Acedo
ACS Applied Materials & Interfaces, 13, 55790-55805 (2021)
Impact Factor (JCR): 10.383 DOI: 10.1021/acsami.1c14592
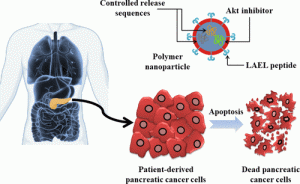
Pancreatic cancer is one of the deadliest cancers partly due to late diagnosis, poor drug delivery to the target site, and acquired resistance to therapy. Therefore, more effective therapies are urgently needed to improve the outcome of patients. In this work, we have tested self-assembling genetically engineered polymeric nanoparticles formed by elastin-like recombinamers (ELRs), carrying a small peptide inhibitor of the protein kinase Akt, in both PANC-1 and patient-derived pancreatic cancer cells (PDX models). Nanoparticle cell uptake was measured by flow cytometry, and subcellular localization was determined by confocal microscopy, which showed a lysosomal localization of these nanoparticles. Furthermore, metabolic activity and cell viability were significantly reduced after incubation with nanoparticles carrying the Akt inhibitor in a time- and dose-dependent fashion. Self-assembling 73 ± 3.2 nm size nanoparticles inhibited phosphorylation and consequent activation of Akt protein, blocked the NF-κB signaling pathway, and triggered caspase 3-mediated apoptosis. Furthermore, in vivo assays showed that ELR-based nanoparticles were suitable devices for drug delivery purposes with long circulating time and minimum toxicity. Hence, the use of these smart nanoparticles could lead to the development of more effective treatment options for pancreatic cancer based on the inhibition of Akt.
“Metronomic anti-cancer therapy: a multimodal therapy governed by the tumor microenvironment“
R. Muñoz, A. Girotti, D. Hileeto, F. J. Arias
Cancers, 13, 5414 (2021)
Impact Factor (JCR): 6.575 DOI: 10.3390/cancers13215414
The concept of cancer as a systemic disease, and the therapeutic implications of this, has gained special relevance. This concept encompasses the interactions between tumor and stromal cells and their microenvironment in the complex setting of primary tumors and metastases. These factors determine cellular co-evolution in time and space, contribute to tumor progression, and could counteract therapeutic effects. Additionally, cancer therapies can induce cellular and molecular responses in the tumor and host that allow them to escape therapy and promote tumor progression. In this study, we describe the vascular network, tumor-infiltrated immune cells, and cancer-associated fibroblasts as sources of heterogeneity and plasticity in the tumor microenvironment, and their influence on cancer progression. We also discuss tumor and host responses to the chemotherapy regimen, at the maximum tolerated dose, mainly targeting cancer cells, and a multimodal metronomic chemotherapy approach targeting both cancer cells and their microenvironment. In a combination therapy context, metronomic chemotherapy exhibits antimetastatic efficacy with low toxicity but is not exempt from resistance mechanisms. As such, a better understanding of the interactions between the components of the tumor microenvironment could improve the selection of drug combinations and schedules, as well as the use of nano-therapeutic agents against certain malignancies.
“Soft Hydrogel Inspired by Elastomeric Proteins“
A. Pepe, L. Maio, A. Bracalello, L. Quintanilla-Sierra, F. J. Arias, A. Girotti, B. Bochicchio
ACS Biomaterials Science & Engineering, 7, 5028-5038 (2021).
Impact Factor (JCR): 5.395 DOI: 10.1021/acsbiomaterials.1c00817
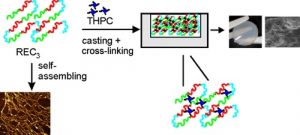
Elastin polypeptides based on -VPGVG- repeated motifs are widely used in the production of biomaterials because they are stimuli-responsive systems. On the other hand, glycine-rich sequences, mainly present in tropoelastin terminal domains, are responsible for the elastin self-assembly. In a previous study, we have recombinantly expressed a chimeric polypeptide, named resilin, elastin, and collagen (REC), inspired by glycine-rich motifs of elastin and containing resilin and collagen sequences as well. Herein, a three-block polypeptide, named (REC)3, was expressed starting from the previous monomer gene by introducing key modifications in the sequence. The choice was mandatory because the uneven distribution of the cross-linking sites in the monomer precluded the hydrogel production. In this work, the cross-linked polypeptide appeared as a soft hydrogel, as assessed by rheology, and the linear un-cross-linked trimer self-aggregated more rapidly than the REC monomer. The absence of cell-adhesive sequences did not affect cell viability, while it was functional to the production of a material presenting antiadhesive properties useful in the integration of synthetic devices in the body and preventing the invasion of cells.
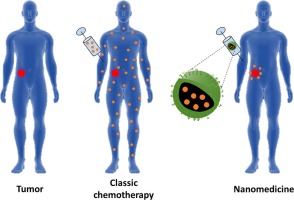
“Advanced nanomedicine and cancer: challenges and opportunities in clinical translation“
J. Gonzalez-Valdivieso, A. Girotti, J. Schneider, F.J. Arias
International Journal of Pharmaceutics, 599, 120438 (2021)
Impact Factor (JCR): 6.510 DOI: 10.1016/j.ijpharm.2021.120438
Cancer has reached pandemic dimensions in the whole world. Although current medicine offers multiple treatment options against cancer, novel therapeutic strategies are needed due to the low specificity of chemotherapeutic drugs, undesired side effects and the presence of different incurable types of cancer. Among these new strategies, nanomedicine arises as an encouraging approach towards personalized medicine with high potential for present and future cancer patients. Therefore, nanomedicine aims to develop novel tools with wide potential in cancer treatment, imaging or even theranostic purposes. Even though numerous preclinical studies have been published with successful preliminary results, promising nanosystems have to face multiple obstacles before adoption in clinical practice as safe options for patients with cancer. In this MiniReview, we provide a short overview on the latest advances in current nanomedicine approaches, challenges and promising strategies towards more accurate cancer treatment.
“Production of elastin-like recombinamer-based nanoparticles for Docetaxel encapsulation and use as smart drug-delivery systems using a supercritical anti-solvent process”
R. Vallejo, J. Gonzalez-Valdivieso, M. Santos, S. Rodriguez-Rojo, F.J. Arias.
Journal of Industrial and Engineering Chemistry, 93, 361-374 (2021)
Impact Factor (JCR): 6.760 DOI: 10.1016/j.jiec.2020.10.013
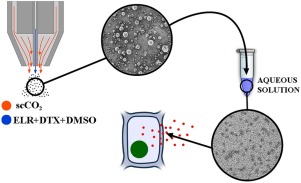
This study presents a new groundbreaking methodology for integrating innovative concepts to develop novel drug-delivery strategies. This methodology combines genetically engineered elastin-like recombinamers (ELRs) with supercritical fluid (SCF) techniques to encapsulate a poorly water-soluble drug in a one-step process. The chemotherapeutic agent docetaxel (DTX) is encapsulated with a block copolymer ELR containing the RGD peptide, a specific target sequence for cancer cells, using the supercritical anti-solvent (SAS) technique in a high process yield of up to 70%. SEM studies show spherical microparticles of 10 μm after encapsulation. After dispersion under physiological conditions, microparticles disaggregate into stable monodisperse nanoparticles of 40 nm size and −30 mV ζ-potential. This protects the drug, as confirmed by NMR analysis, thereby increasing the water solubility of DTX up to fifty orders of magnitude. The delivery process is governed by the Fick diffusion mechanism and indicates that the presence of DTX on the particles surface is practically negligible. Cellular assays showed that, due to the presence of the cancer target sequence RGD, breast cancer cells were more affected than human endothelial cells, thus meaning that the strategy developed in this work opens the way to new controlled release systems more precise than non-selective chemotherapeutic drugs.
2020
“Aptamer-Functionalized Natural Protein-based Polymers as Innovative Biomaterials”
A. Girotti, S. Escalera Anzola, I. Alonso Sampedro, J. Gonzalez Valdivieso, F.J. Arias.
Pharmaceutics, 12, 1115 (2020)
Impact Factor (JCR): 6.321 DOI:10.3390/pharmaceutics12111115
Biomaterials science is one of the most rapidly evolving fields in biomedicine. However, although novel biomaterials have achieved well-defined goals, such as the production of devices with improved biocompatibility and mechanical properties, their development could be more ambitious. Indeed, the integration of active targeting strategies has been shown to allow spatiotemporal control of cell–material interactions, thus leading to more specific and better-performing devices. This manuscript reviews recent advances that have led to enhanced biomaterials resulting from the use of natural structural macromolecules. In this regard, several structural macromolecules have been adapted or modified using biohybrid approaches for use in both regenerative medicine and therapeutic delivery. The integration of structural and functional features and aptamer targeting, although still incipient, has already shown its ability and wide-reaching potential. In this review, we discuss aptamer-functionalized hybrid protein-based or polymeric biomaterials derived from structural macromolecules, with a focus on bioresponsive/bioactive systems.
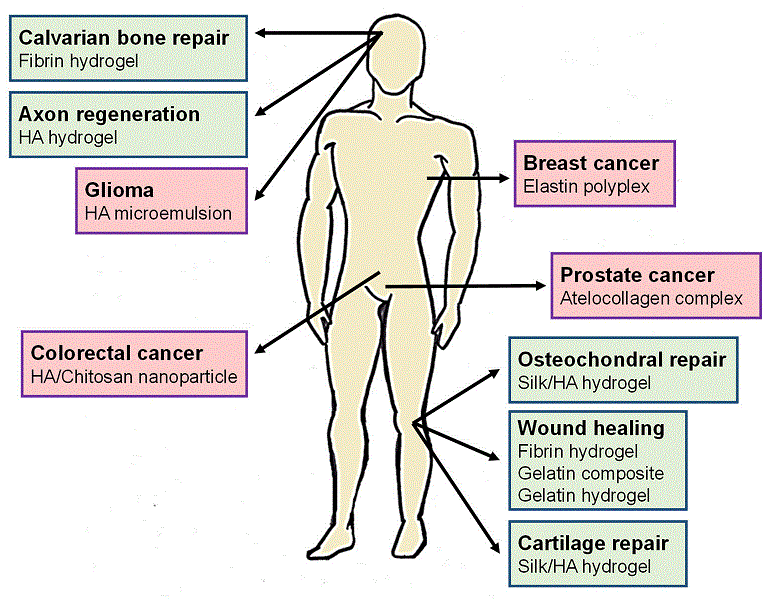
“Functional characterization of an enzymatically degradable multi-bioactive Elastin-Like Recombinamer”
A. Girotti, J. Gonzalez-Valdivieso, M. Santos, L. Martin, F.J. Arias
International Journal of Biological Macromolecules, 164, 1640-1648 (2020)
Impact Factor (JCR): 6.953 DOI: 10.1016/j.ijbiomac.2020.08.004
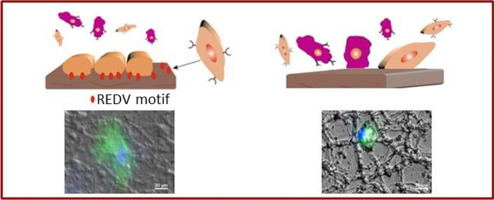
One of the main goals in both tissue engineering and regenerative medicine is to design innovative synthetic scaffolds that can simulate and control the communication pathways between cells and the extracellular matrix (ECM). In this context, we describe herein the characterization of protein polymer, a recombinant elastin-like recombinamer (ELR) designed for developing tissue-engineered devices for use in vascular regeneration. This ELR is composed of an elastin-like backbone that contains a fibronectin domain, which provides specific, endothelial cell adhesion, and a protease target domain directed towards specific proteases involved in ECM remodeling. We also compare the specific response of endothelial and fibroblast cells to ELR scaffolds and show that cell adhesion and spreading on this ELR is significantly higher for endothelial cells than for fibroblasts. The reactivity of this polymer and its hydrogels to specific enzymatic degradation is demonstrated in vitro. As with natural elastin, enzymatic hydrolysis of the ELR produces elastin-derived peptides, or “matrikines”, which, in turn, are potentially able to regulate important cell activities.
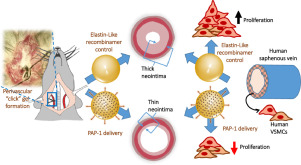
“Elastin-like recombinamer-based devices releasing Kv1.3 blockers for the prevention of intimal hyperplasia: an in vitro and in vivo study”.
S. Moreno-Estar, S. Serrano, M.C. Arévalo-Martinez, P. Cidad, J.R. López-López, M. Santos, M. T. Pérez-García, F.J. Arias
Acta Biomaterialia, 115, 264-274 (2020)
Impact Factor (JCR): 8.947 DOI: 10.1016/j.actbio.2020.07.053
Coronary artery disease (CAD) is the most common cardiovascular disorder. Vascular surgery strategies for coronary revascularization (either percutaneous or open) show a high rate of failure because of restenosis of the vessel, due to phenotypic switch of vascular smooth muscle cells (VSMCs) leading to proliferation and migration. We have previously reported that the inhibition of Kv1.3 channel function with selective blockers represents an effective strategy for the prevention of restenosis in human vessels used for coronary angioplasty procedures. However, delivery systems for controlled release of these drugs have not been investigated. Here we tested the efficacy of several formulations of elastin like recombinamers (ELRs) hydrogels to deliver the Kv1.3 blocker PAP-1 in various restenosis models. The dose and time course of PAP-1 release from ELRs click hydrogels was able to inhibit human VSMC proliferation in vitro as well as remodeling of human vessels in organ culture and restenosis in in vivo models. We conclude that this combination of active compound and advanced delivery method could improve the outcomes of vascular surgery in patients.
“A DNA vaccine delivery platform based on Elastin-Like Recombinamer nanosystems for Rift Valley fever virus”
J. Gonzalez-Valdivieso, B. Borrego, A. Girotti, S. Moreno, A. Brun, J. Bermejo-Martin, F.J. Arias
Molecular Pharmaceutics, 17, 1608-1620 (2020)
Impact Factor (JCR): 4.939 DOI: 10.1021/acs.molpharmaceut.0c00054
This work analyzes the immunogenicity of six genetically engineered constructs based on elastin-like recombinamers (ELRs) fused to the Gn glycoprotein from Rift Valley fever virus (RVFV). Upon transfection, all constructs showed no effect on cell viability. While fusion constructs including ELR blocks containing hydrophobic amino acids (alanine or isoleucine) did not increase the expression of viral Gn in eukaryotic cells, glutamic acid- or valine-rich fusion proteins showed enhanced expression levels compared with the constructs encoding the viral antigen alone. However, in vivo DNA plasmid immunization assays determined that the more hydrophobic constructs reduced viremia levels after RVFV challenge to a higher extent than glutamic- or valine-rich encoding plasmids and were better inducers of cellular immunity as judged by in vitro restimulation experiments. Although the Gn-ELR fusion constructs did not surpass the protective efficacy of a plasmid vaccine expressing nonfused Gn, our results warrant further experiments directed to take advantage of the immunomodulatory potential of ELR biomaterials for improving vaccines against infectious diseases.
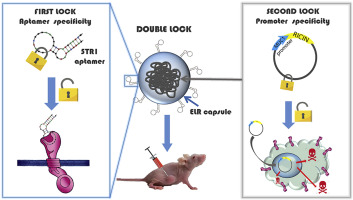
“A Double Safety Lock Tumor-Specific Device for Suicide Gene Therapy in Breast Cancer”
M.J. Piña, A. Girotti, S. Serrano, R. Muñoz, J. C. Rodriguez-Cabello, F.J. Arias.
Cancer Letters, 470, 43-53 (2020)
Impact Factor (JCR): 8.679 DOI: 10.1016/j.canlet.2019.11.031
The complexity and continuous evolution of cancer make the design of novel strategies of treatment a constant challenge in biomedicine. Moreover, most of cancer treatments are still not tumor-specific and provoke high systemic toxicity. Herein we have developed a novel selective nanodevice to eliminate tumor cells while leaving healthy ones intact. To achieve this objective, a polyplex carrier, comprising an elastin like-recombinamer covalently conjugated to an aptamer and complexed with therapeutic DNA, was tested. This carrier forms a double-lock multifunctional device due to specific binding to a tumor cell marker and the selective expression of therapeutic DNA inside human breast-cancer cells. Due to the stability provided by ELRs, the homogeneous population of polyplexes obtained showed selective toxicity against cancer cells in in vitro and in vivo assay. Inhibition of tumor progression was detected early being very significant at the end point, with a dose-dependent reduction in tumor mass. Histological studies revealed a specific reduction in tumor parenchyma and in specific tumor cell markers. These results represent an important step toward the rational development of an efficient, safe and more specialized gene-delivery device for tumor therapy.